Speed by Default.
Quality by Design.
The new standard for Sponsors, CROs and Institutions to run faster trials.
Instant adoption. Human-in-the-loop AI. Zero compliance risk.

Carelane is an AI-driven platform engineered to accelerate Phase I-IV trials, registries, and other clinical research. We unify EDC, ePRO, Consent Management, CTMS, and more into a single, high-speed infrastructure. By operating on a single source of truth, sponsors and CROs eliminate manual friction and slash study timelines by 70%.
Carelane by the Numbers
Impact you can measure.
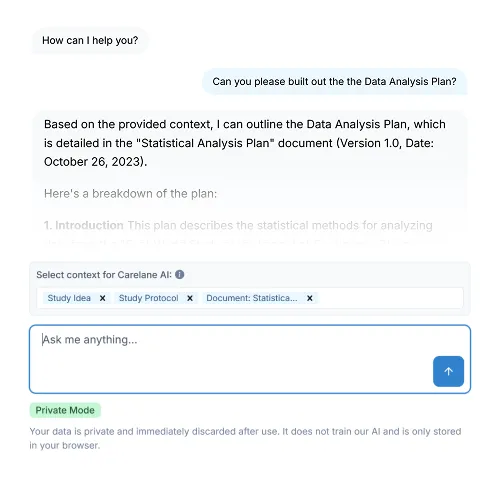
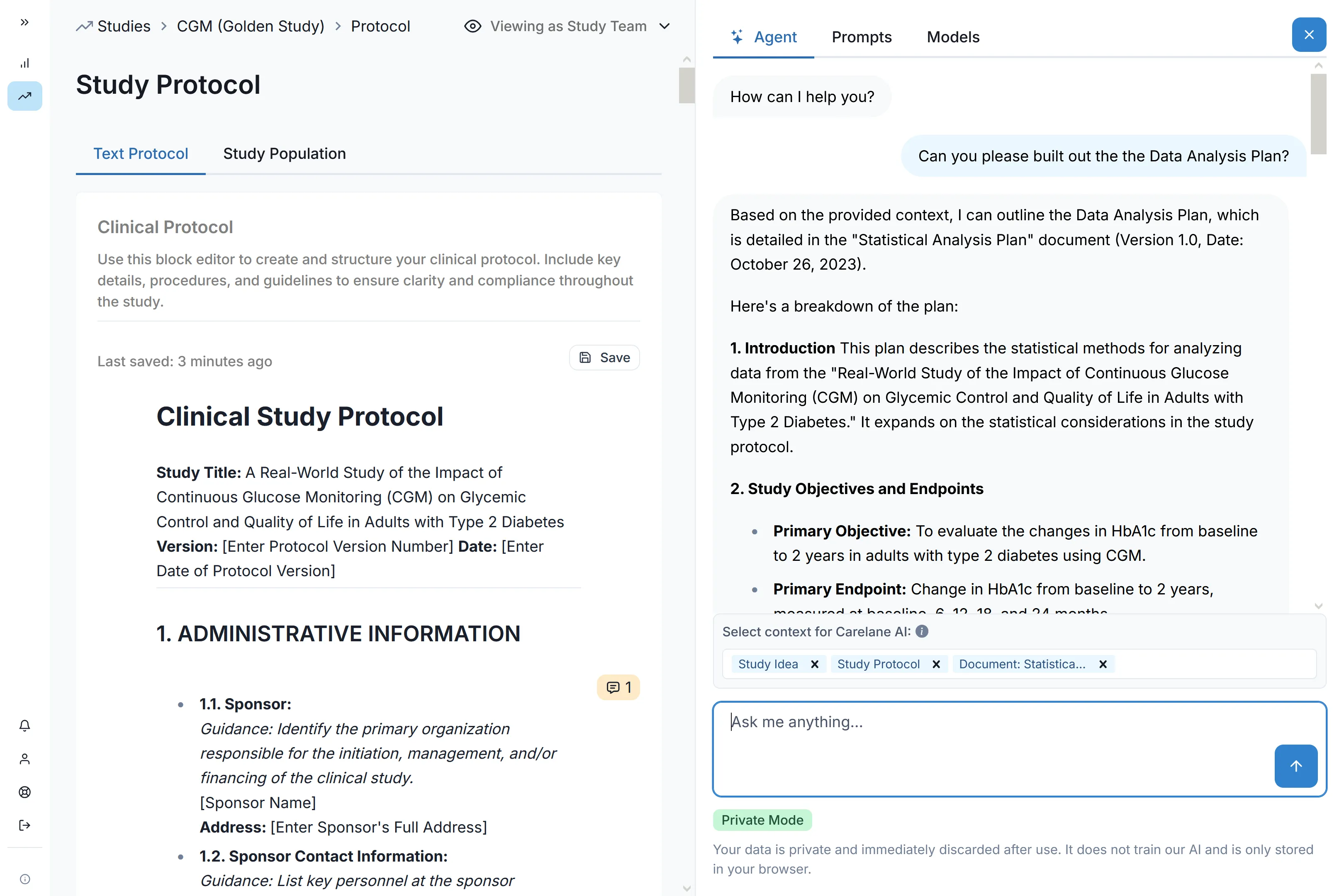
Digital Protocol Development
Structured FHIR protocols synchronise automatically across systems, accelerate amendments through version control, and enable advanced direct data capture via standardised interoperability.
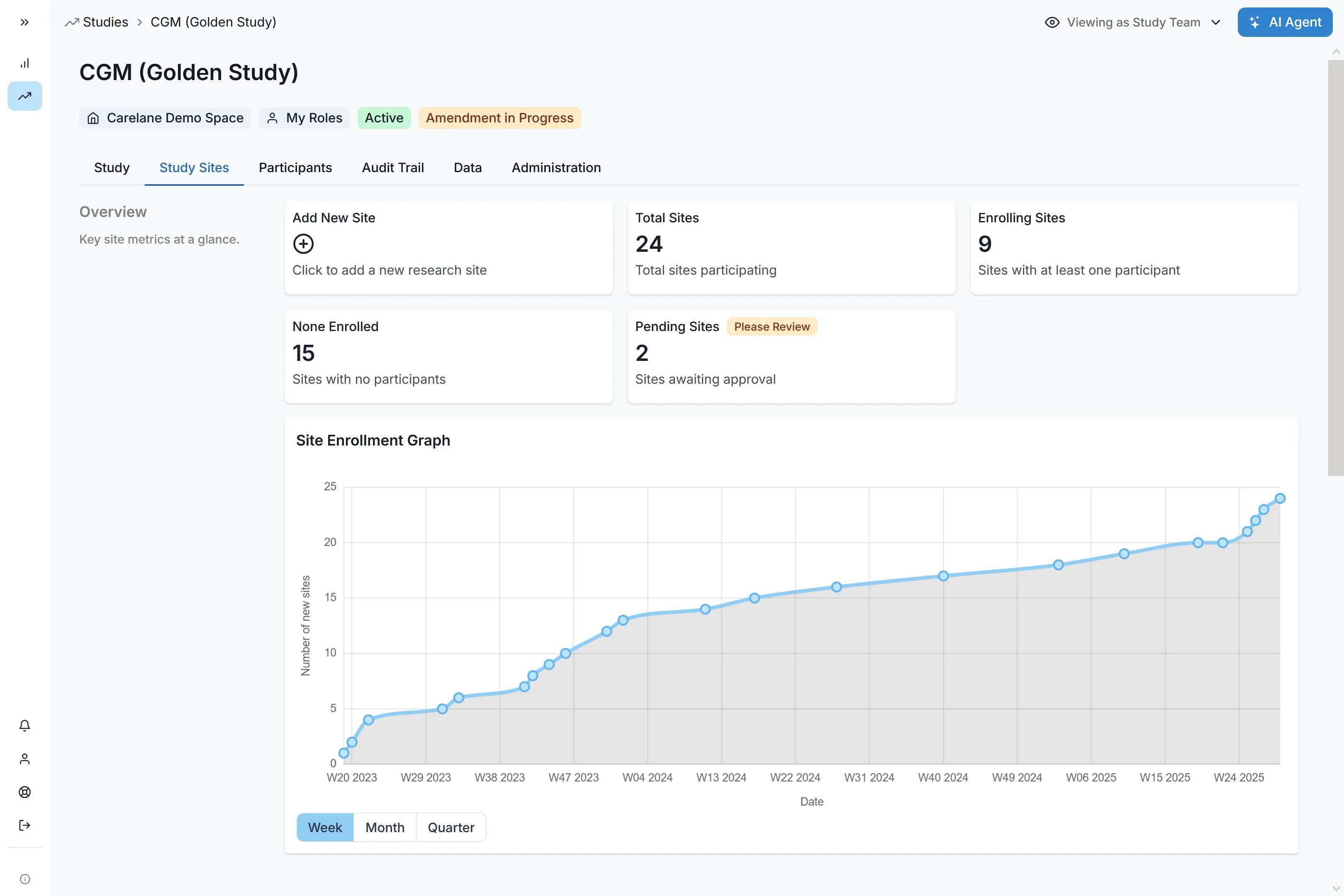
Site Data
Entry
Process-first workflows and user-friendly interface reduce data entry time from 6 to 1 hour per patient.
Sites utilising Carelane report superior satisfaction due to the platform's ease of use.
Site Support & Communication
Real-time data visibility and integrated platform reduce support effort from 20 person-days to 1 person-day.
Total Study
Costs
Streamlined consents, simplified workflows, and reduced practitioner burden double participant numbers.
Query
Management
Intelligent edit checks minimise queries while AI-assisted resolution reduces response time from weeks to days.
Site Selection & Self-Registration
Automated self-registration workflows and integrated feasibility assessments reduce site selection from 10 weeks to 1 week.
CRF Design
AI aligns objectives with outcome measures and leverages pre-validated libraries to reduce design time from 6 weeks to 1 week.
Recruitment
Streamlined consents, simplified workflows, and reduced practitioner burden enable enrollment of more than twice the participants.
Patient Registries
Launch and sustain patient registries that grow from dozens to thousands of participants across countries—with infrastructure designed for long-term viability, not just short-term studies.
Academic Research
Conduct investigator-initiated trials with institutional-quality infrastructure designed for research teams that need to launch quickly and operate efficiently, without the traditional cost barriers.
Phase IV Trials
Launch Phase IV trials in weeks, not quarters. Purpose-built infrastructure for post-marketing studies that need to move quickly, integrate seamlessly, and deliver high-quality RWE without interventional trial overhead.
Observational Studies
Every observational study is unique. Your platform shouldn't force you into rigid templates. Launch cohort studies, case-control studies, pragmatic trials, and cross-sectional research with infrastructure that adapts to your design—not the other way around.
Fortified by Design: Your Data, Our Priority
Impenetrable PHI Encryption
We secure your PHI with zero-knowledge encryption. Each piece of data has a unique key, isolated by study and site. Only authorised site members hold the keys - not Carelane, not sponsors.
Precision Access,
Perfect Control (RBAC)
We use Role-Based Access Controls (RBAC) to meticuously sculpt user's access on a strictly need-to-know basis. With Carelane you gain surgical control over data visibility.
Architectural Data Isolation
We architect unique, segregated data collections for every client, study, and site. Encryption keys are stored in a separate key-fortress, ensuring your information is uniquely secure.
Ready to try Carelane?
The new standard for Sponsors, CROs and Institutions to run faster trials. Instant adoption. Human-in-the-loop AI. Zero compliance risk.