Audit-Ready Clinical Trials, launched in days.
The Operating System for Sponsors, CROs, and Institutions.
“The study setup was highly intuitive and the time we saved on site selection and onboarding was impressive. By adapting the Carelane workflow templates and adding them to our study setup, we only needed to send out one email; everything else was automated.”
Medical University of Graz
Platform-Wide Intelligence
AI-Powered Assistance
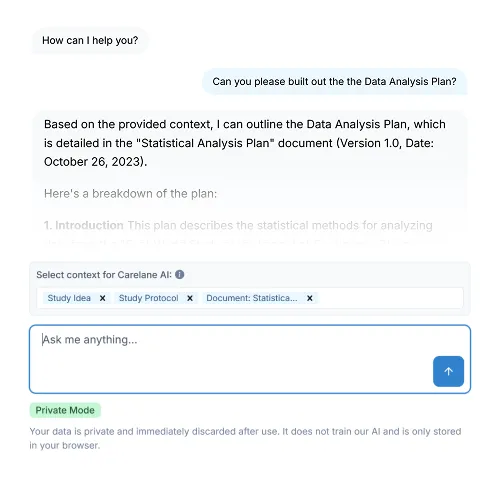
AI Agent:
Your always-on research assistant. Automate artifact alignment, accelerate data review, and resolve issues instantly via chat.AI CRF Builder:
Turn concepts into CRFs in seconds. Our AI defines data elements and applies metadata to standardise collection automatically.
Collaboration Hub
Unified Platform:
Unified Platform: One login, one source of truth. Teams collaborate in real-time with project management tools deeply integrated into the study data.
Study Design
Digital Protocol Development
Protocol Digitisation:
Start translating your static Word docs to structured, digital workflows that sync automatically across the platform.AI-Powered Design:
Use simple prompts to generate complex protocol elements, coordinate changes, and ensure consistency.Cohort & Endpoint Definition:
Precisely structure patient populations and outcome measures to ensure downstream data validity.
Planning & Standardization
Statistical Analysis Plan (SAP):
Instantly generate standardised SAPs that link directly to your digitised objectives and data definitions.Schedule of Activities (SoA):
Visualise and control assessment timelines to ensure site compliance and consistency.Template Management:
Don't start from scratch. Leverage pre-validated libraries and your own templates to launch studies faster.
Study Setup
Site Management
Site Network Management:
Reduce administrative drag. Centralise communication and relationship tracking for all your sites.Site Self-Registration:
Zero-touch onboarding. Allow sites to register themselves via guided, automated workflows.Onboarding & Training:
Automate the busy work. Manage role delegation, remote initiation, and training tracking in one flow.
Feasibility & Ethics
Capabilities and Feasibility:
Digital workflows ensure sites are qualified and equipped before you activate them.Ethics/IRB Submission Ready:
Streamline approvals with a dedicated hub that centralises all essential documents for review boards.
Study Conduct
Participant Management
Full Participant Lifecycle:
Seamlessly manage the patient journey from prescreening to final follow-up.Randomisation & Tokenisation:
Integrated randomisation that maintains scientific rigor while strictly preserving privacy via tokenisation.
Data Capture & Management
eSource (Data Capture & SDC):
Capture source data directly at the point of care. Flexible, verified inputs eliminate transcription errors and make data instantly available for review.
(COMING SOON)Interoperability:
Seamlessly exchange data with EHRs and external systems. Built on native FHIR standards and clinical terminologies (SNOMED, LOINC) to ensure your study speaks the language of modern healthcare.
(COMING SOON)eCOA (electronic Clinical Outcome Assessment):
Replace paper with precision. Collect clinical outcome data electronically to ensure higher data integrity, better compliance, and real-time visibility.
ePRO (electronic Patient-Reported Outcome):
Empower patients to participate from anywhere. Securely capture data directly from patients' own devices to improve retention and data richness.
(COMING SOON)Embedded Protocols:
Bridge the gap between research and clinical care. Use FHIR workflow resources to align study events with standard medical procedures, reducing burden on site staff.
Data Management Suite:
Keep your data clean automatically. Configure built-in checks, auto-calculations, and streamlined review processes to catch errors the moment they happen.
Query Management:
Slash data cleaning time. AI-assisted workflows automate query generation, routing, and tracking, helping teams resolve issues in days rather than weeks.
Monitoring & Analytics
Monitoring & Analytics
Remote Monitoring:
Give monitors secure, temporary access to verify data integrity without compromising PHI security.
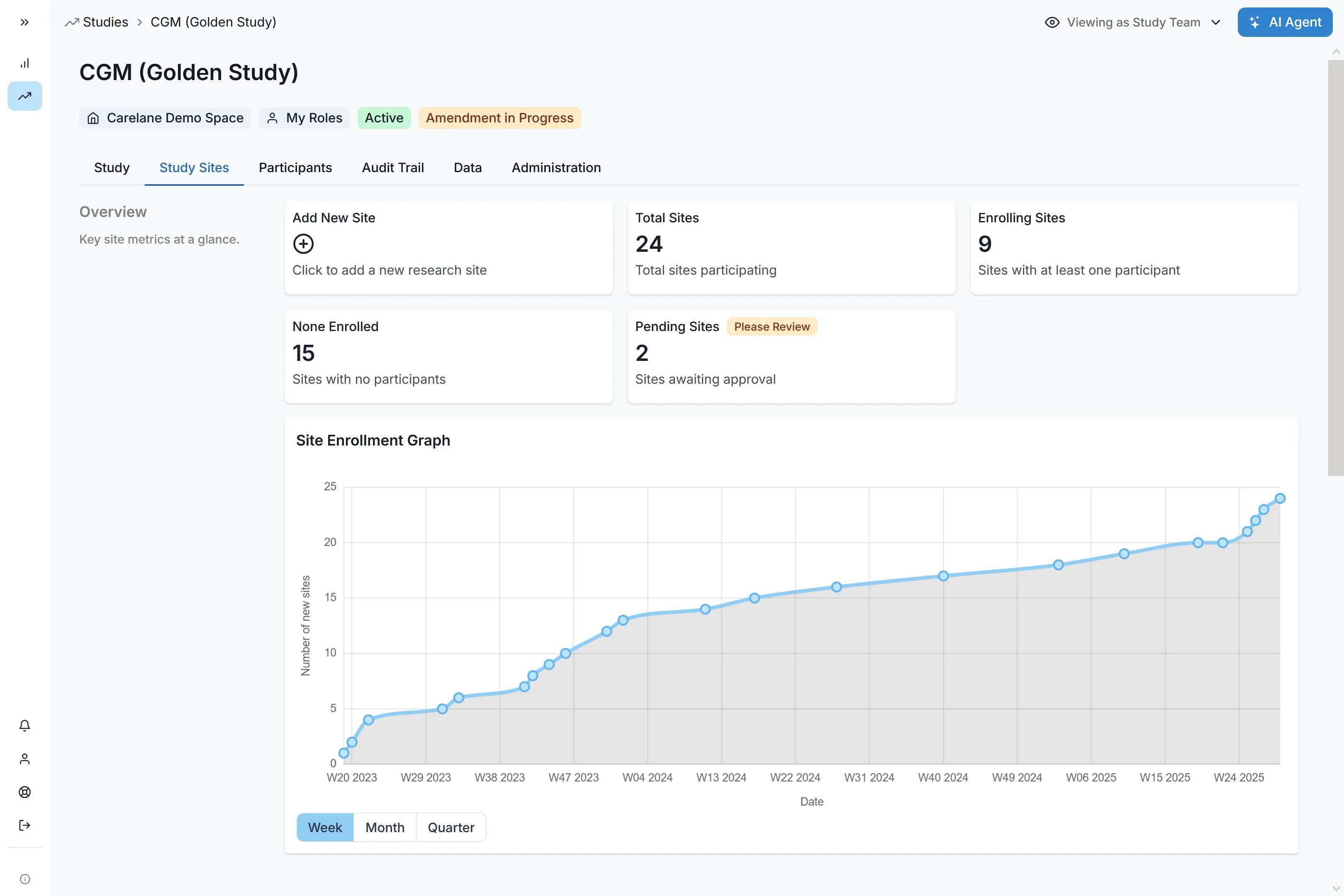
Reporting & Analytics:
Real-time dashboards track KPIs and enrolment metrics instantly.
Custom Study Reports:
Flexible data grids let you filter, pivot, and group data to generate the exact insights you need.
Data Export:
Export in native FHIR format for seamless integration with external analytics tools (SAS, R, etc.).
Security & Trust
Advanced Encryption
Granular PHI Encryption (Zero-Knowledge):
We use a unique encryption key for every single site in every single study. Only authorized site members can access the keys—not Carelane,, not the CRO and not the Sponsor.
Encryption in Transit & At Rest:
Comprehensive protection for data in motion and in storage.
General Data Encryption:
Certified protection (ISO 27001, SOC 2) for all study data.
Access & Control
Role-Based Access Controls (RBAC):
Strict "Principle of Least Privilege" ensures users see only what they need to see.
Data Isolation:
Unique, segregated data collections for every client and study prevent data crossover.
Secure Key Management:
Encryption keys are stored in a "Key Fortress"—physically and logically separated from the data itself.
Compliance & Logging
Continuous Auditing & Logging:
A rigorous, immutable log of every access event and modification ensures full traceability (21 CFR Part 11 compliant).
Real-time Threat Monitoring:
Carelane leverages active detection systems to protect you and your organization’s data.
Ready to try Carelane?
The new standard for Sponsors, CROs and Institutions to run faster trials. Instant adoption. Human-in-the-loop AI. Zero compliance risk.