Pricing
Simple and transparent, built for scale.
Academic Research
Business
Enterprise
Full platform access for reduced budgets. Launch investigator-initiated trials without the commercial overhead.
Includes everything from "Academic Research" plus unlimited customer support.
Your end-to-end solution for Observational Clinical Research. Unlimited AI-requests and support.
Key Considerations (subject to fair usage policy)
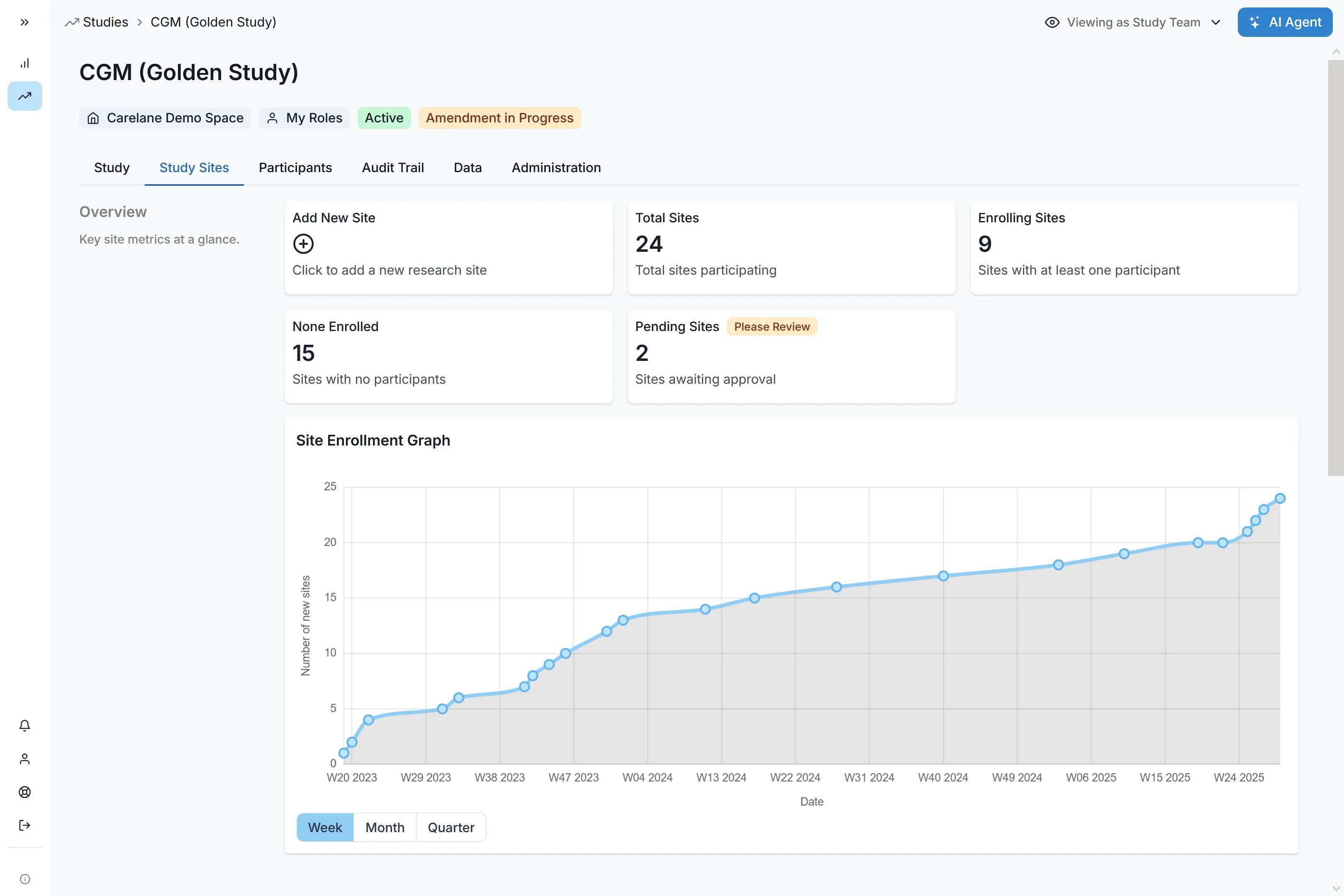
Nr. Sites
Nr. Users
Nr. Participants
Nr. Draft Studies
Tier Differentiators
Nr. AI-Requests
High Usage Limit
High Usage Limit
Support
Limited
Platform Wide Features
AI Agent
AI CRF Builder
Unified Platform
Study Design
Protocol Digitization
AI-Powered Design
Cohort Definition
Endpoint Definition
Statystical Analysis Plan
Schedule of Activities
Template Management
Ethics/IRB Submission Ready
Study Setup
Site Network Management
Site Self-Registration
Site Onboarding & Training
Capabilities and Feasibility Workflows
Protocol Amendments
Study Conduct
Full Participant Lifecycle
Randomisation & Tokenisation
eSource (Data Capture & SDC) (coming soon)
Interoperability
ePRO (coming soon)
Embedded Protocols
Data Management Suite
Query Management
Remote Monitoring
Reporting & Analytics
Custom Study Reports
Data Export
Security & Trust
Encryption in Transit & At Rest
Granular PHI Encryption
General Data Encryption
Role-Based Access Controls (RBAC)
Continuous Auditing
Data Isolation
Secure Key Management
Real-time Monitoring
Detailed Access & Change Logging
2 Factor Authentication
Compliance
SOC2
HIPAA
GDPR
CFR 21 part 11
Academic Research
Full platform access for reduced budgets. Launch investigator-initiated trials without the commercial overhead.
Business
Includes everything from "Academic Research" plus unlimited customer support.
Enterprise
Your end-to-end solution for Observational Clinical Research. Unlimited AI-requests and support.
Ready to try Carelane?
The new standard for Sponsors, CROs and Institutions to run faster trials. Instant adoption. Human-in-the-loop AI. Zero compliance risk.