Accelerate Evidence Generation. Launch Phase IV in Weeks, Not Quarters.
Stop waiting on legacy infrastructure. Carelane automates the transition from protocol to patient data, enabling site activation and evidence generation at the speed of your commercial launch.
Carelane accelerates Phase IV trials and Post-Marketing Surveillance through AI-powered automation and FHIR-standard digitisation. By streamlining site activation and data collection, the platform reduces study timelines by up to 90%. This high-speed infrastructure enables sponsors to generate real-world evidence rapidly, ensuring faster market insights and regulatory compliance at a fraction of traditional costs.
Phase IV Demands Speed Without Compromise
Post-marketing studies face unique pressures: commercial launch timelines, real-world setting complexity, and the need for rapid, high-quality evidence generation. Traditional trial infrastructure wasn't built for this.
Purpose-Built Infrastructure for Phase IV
Carelane delivers Clinical Trial Software optimised for Phase IV requirements: rapid deployment, real-world pragmatism, and seamless integration with existing healthcare infrastructure.

Digital Protocols Integrated with Routine Care
Deploy FHIR-based digital protocols that integrate directly with clinical workflows. Rather than creating parallel research systems, Carelane aligns study activities with routine care, reducing site burden by 50-85% and improving protocol compliance.

Rapid Multi-Site Deployment
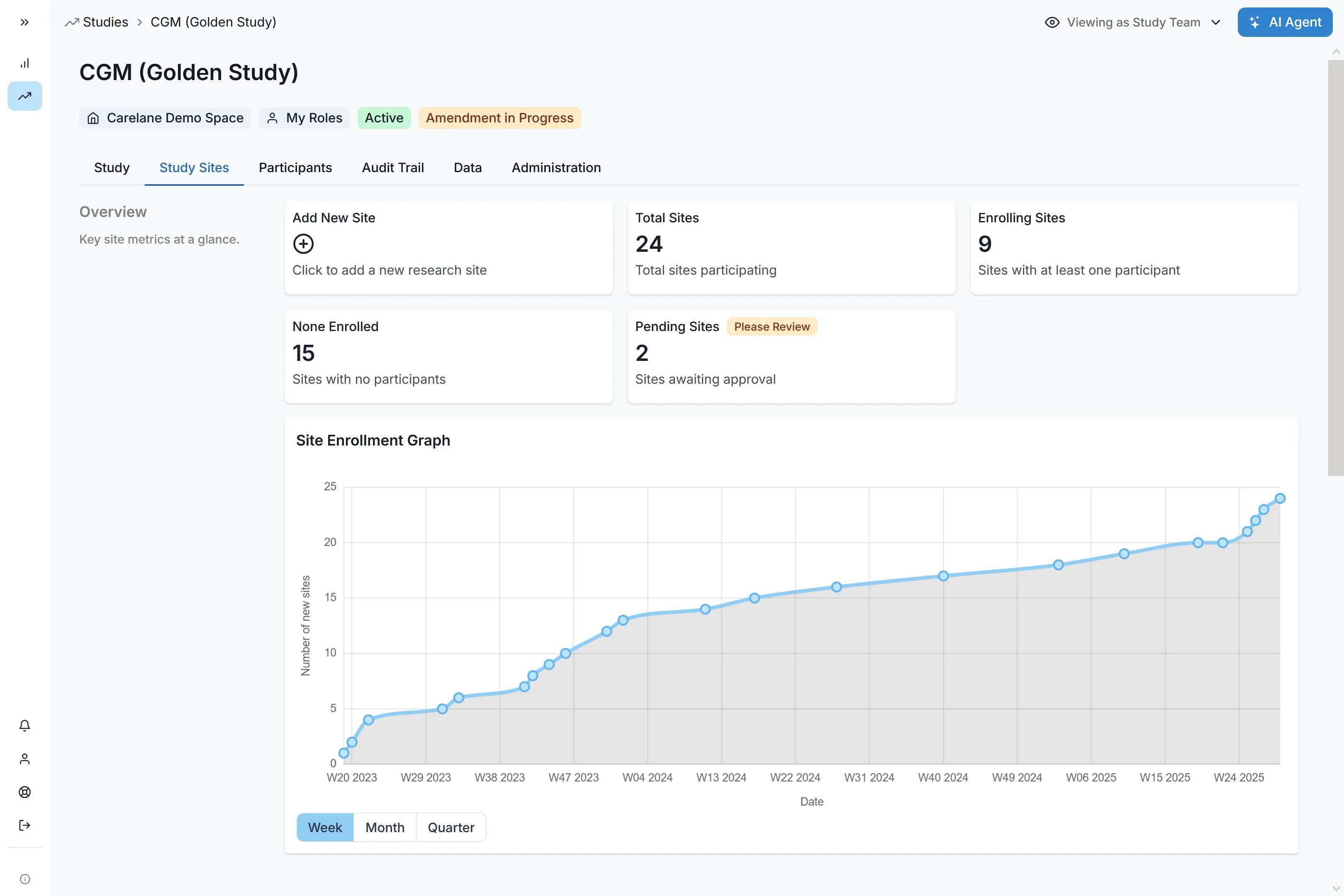
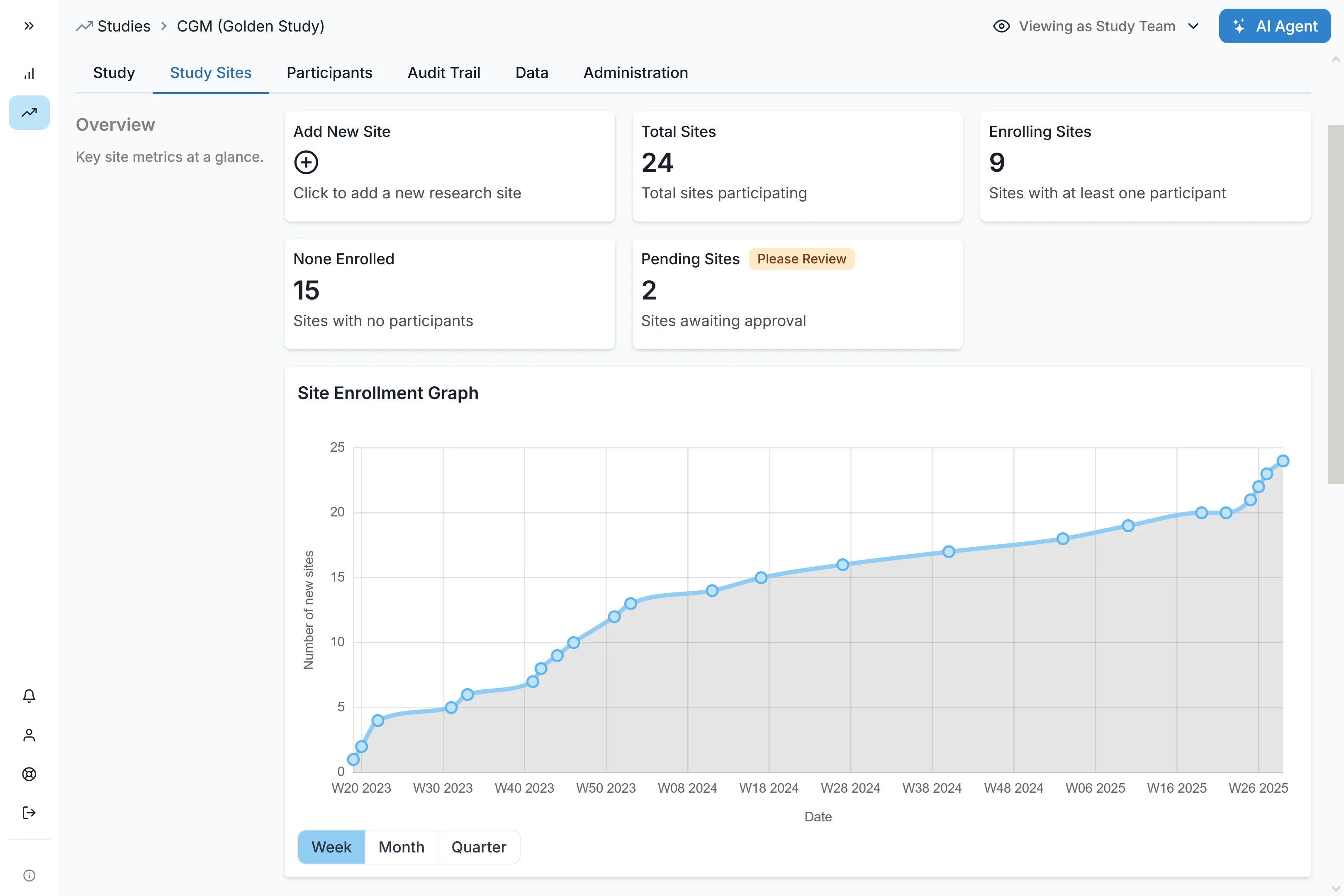
Launch Phase IV programs with unprecedented speed. Activate up to 60 sites in a single week across multiple countries, complete with capability assessment and onboarding. Reduce site selection time through intelligent feasibility workflows, ensuring your evidence generation stays ahead of commercial timelines.

FHIR Interoperability and Standards-Based Data Exchange
Seamlessly integrate with existing healthcare IT infrastructure using FHIR standards and clinical terminologies (ICD-10, SNOMED, LOINC). Eliminate duplicate data entry, leverage existing EHR data, and enable automated data flows that reduce manual effort by 70-95% while maintaining data quality and regulatory compliance.

Pragmatic Trial Design Support
Optimise for real-world evidence generation with flexible enrolment criteria, minimal exclusions, and adaptive data collection. Reduce EDC setup time by 70-90% through libraries of eCRFs and automated configuration tools designed specifically for observational research requirements.
Proven Performance Across Post-Marketing Programs
CROs and pharmaceutical sponsors are leveraging Carelane to accelerate Phase IV execution, reduce operational costs, and deliver regulatory-grade RWE faster.
Real-World Evidence is Redefining Drug Development
Regulatory bodies, payers, and healthcare systems increasingly rely on post-marketing evidence for coverage and formulary decisions. Speed to quality RWE generation directly impacts commercial success.
Regulatory Evolution
FDA, EMA and international regulators now accept real-world evidence for label expansions, safety updates, and comparative effectiveness claims. Phase IV studies can generate evidence that directly enhances product value—if executed quickly enough to matter.
Competitive Differentiator
In crowded therapeutic areas, high-quality RWE distinguishes products from competitors. Organisations that generate Phase IV evidence faster gain strategic advantages in market positioning and clinical guideline influence.
Operational Efficiency
Phase IV budgets face increasing scrutiny. Studies using interventional-grade infrastructure for observational research waste resources on unnecessary complexity. Purpose-built observational tools enable better quality at lower costs.
Ready to try Carelane?
The new standard for Sponsors, CROs and Institutions to run faster trials. Instant adoption. Human-in-the-loop AI. Zero compliance risk.