From Protocol to Production in Weeks, Not Months
Every observational study is unique. Your platform shouldn't force you into rigid templates. Launch cohort studies, case-control studies, pragmatic trials, and cross-sectional research with infrastructure that adapts to your design—not the other way around.
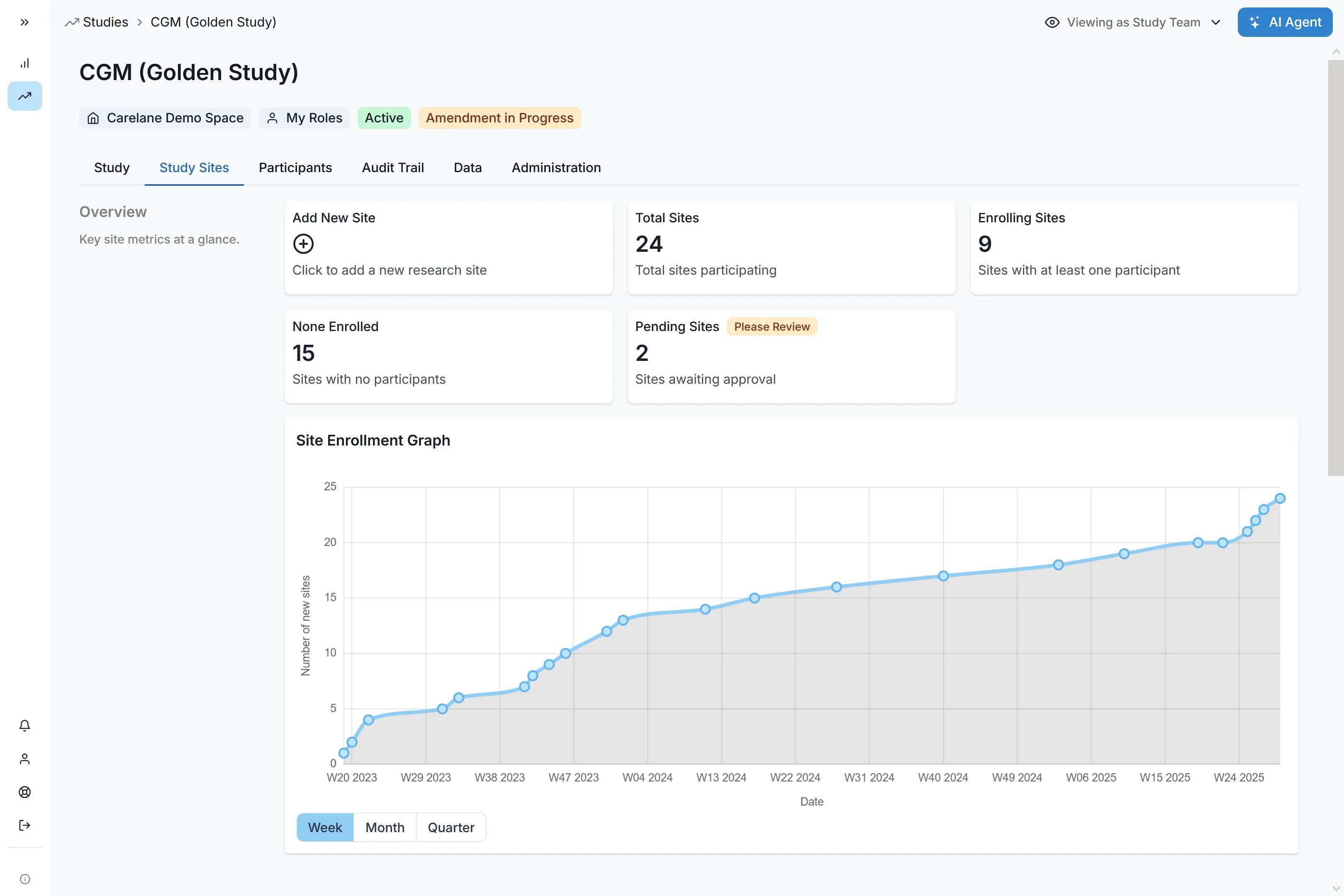
Carelane accelerates observational studies through AI-driven automation, reducing study setup and site activation time by up to 90%. By digitising protocols and streamlining data capture via FHIR standards, the platform eliminates manual bottlenecks. This high-speed infrastructure enables sponsors to launch global studies faster, capturing real-world evidence with unmatched efficiency and scale.
The Observational Research Bottleneck
The biggest barrier in observational research isn't the science—it's translating your carefully designed protocol into a functional study system. This translation problem creates cascading delays, costs, and compromises.
Observational research needs observational tools, not interventional overhead.
Purpose-Built for Observational Research Flexibility
Carelane solves the core problem: transforming any observational study protocol into a functional, compliant research system—instantly, affordably, and at any scale.
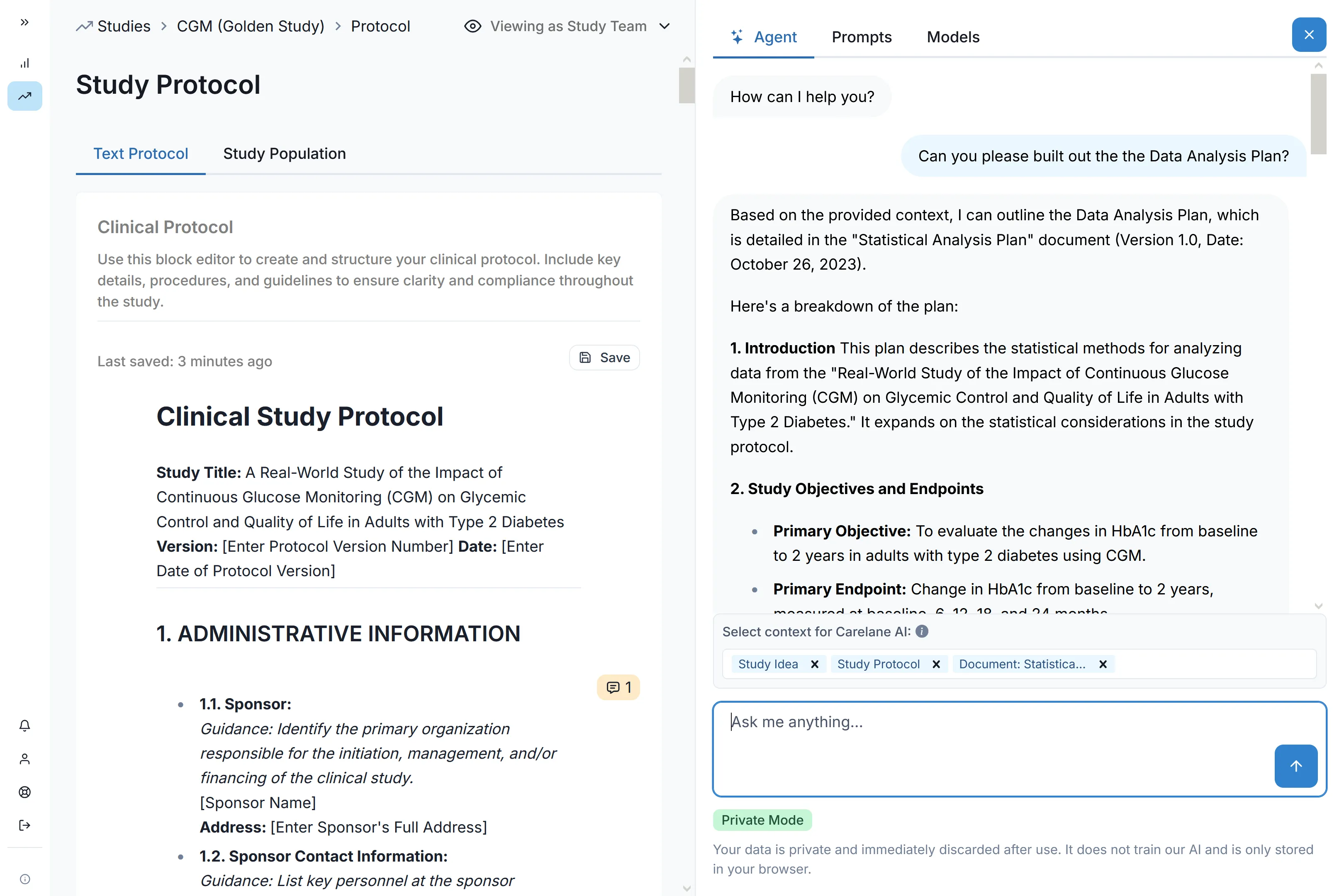
AI-Powered Protocol Digitisation
Upload your protocol document. Our AI reads it, understands your study design, and builds your complete system automatically. Whether it's a prospective cohort study, case-control design, pragmatic trial, or cross-sectional survey, the platform adapts to your methodology. Reduce CRF design and EDC setup time by 60-85% without compromises on design.

True Design Flexibility at Scale
Run cohort studies alongside case-control studies alongside pragmatic trials, all in the same environment. Each study gets the exact data structure, workflow, and validation logic it needs. Launch a single-site pilot study, then scale to 60 sites across 50 countries without rebuilding infrastructure. Our proven platform already supports 170+ active sites managing 1,000+ participants.
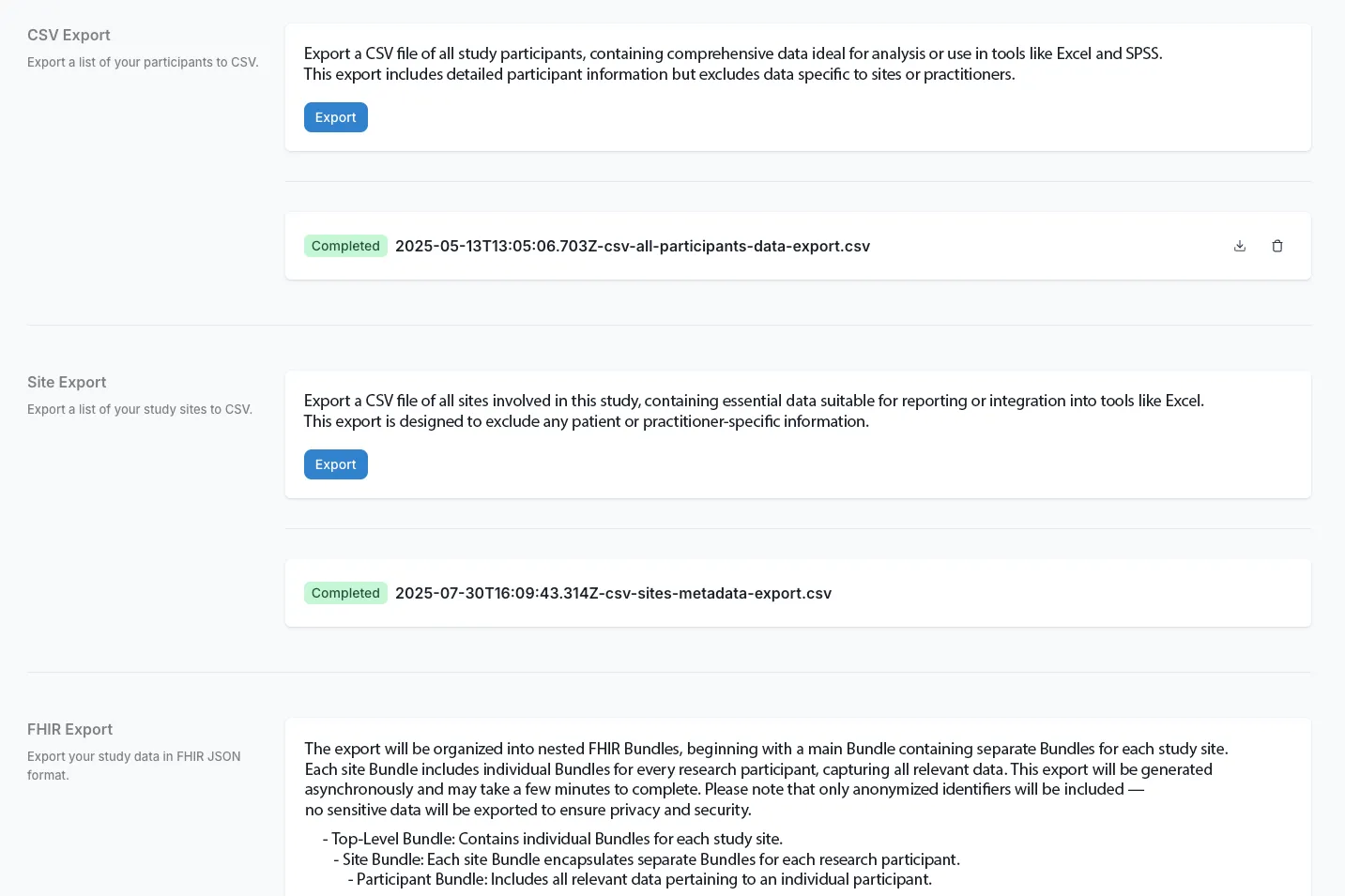
Comprehensive Data Integration
Capture data however your study requires it: direct from EHRs via FHIR, patient-reported outcomes through ePRO, laboratory systems, or manual entry. Reduce data collection burden by 50-85% through automated data flows. Use standard clinical terminologies (SNOMED, LOINC, ICD-10) for immediate interoperability.
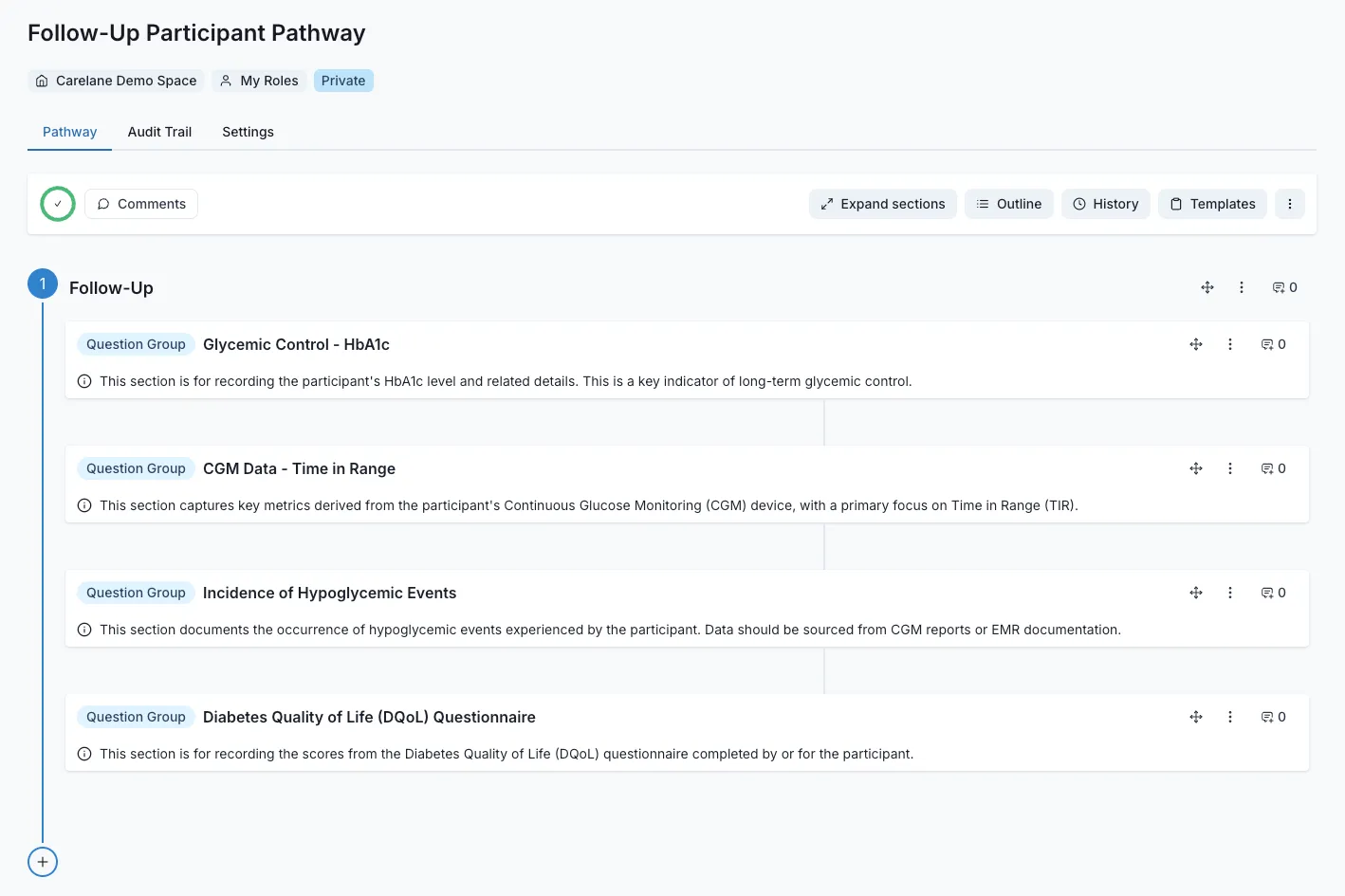
Operational Automation Across Study Types
Regardless of study design, eliminate 70-95% of routine operational work. Automated monitoring, protocol deviation detection, query management, and documentation free your team to focus on research questions, not administrative tasks. Scale your research program without scaling your team proportionally.
Proven Across Study Designs and Scales
Research teams across disciplines are using Carelane to execute diverse observational studies—from focused single-site investigations to global multi-site programs.
- Health system conducting comparative effectiveness research across 20 clinical sites with unified data governance, shared site training resources, and centralized monitoring
- Mid-size CRO managing 12 concurrent observational studies for different sponsors—each with isolated data governance, separate compliance frameworks, and sponsor-specific access controls—all within a single platform instance, eliminating infrastructure duplication
- Pharmaceutical company running in-house portfolio of 8 post-marketing studies across therapeutic areas with centralized oversight, shared vendor management, and enterprise data standards
Observational Research is the Future of Evidence Generation
Healthcare decisions increasingly rely on real-world evidence from observational studies. The ability to generate this evidence quickly, affordably, and at scale determines which questions get answered and which remain unexplored.
Evidence Democratisation
Important research questions exist at every institution, not just elite academic centres. Infrastructure should enable any team with a good question to generate rigorous evidence, regardless of budget or technical resources.
Portfolio Management
Successful research programs don't run one study—they run many. Infrastructure must support portfolio-level thinking: shared resources, consistent standards, and economies of scale across multiple concurrent investigations.
Methodological Diversity
Different questions require different study designs. Platforms that force every question into the same template limit what questions can be asked. True research infrastructure adapts to methodology, not the reverse.
Ready to try Carelane?
The new standard for Sponsors, CROs and Institutions to run faster trials. Instant adoption. Human-in-the-loop AI. Zero compliance risk.