Enterprise-Grade Tools for Every Researcher
Launch quickly and operate efficiently, without the traditional cost barriers of top-shelf tools.
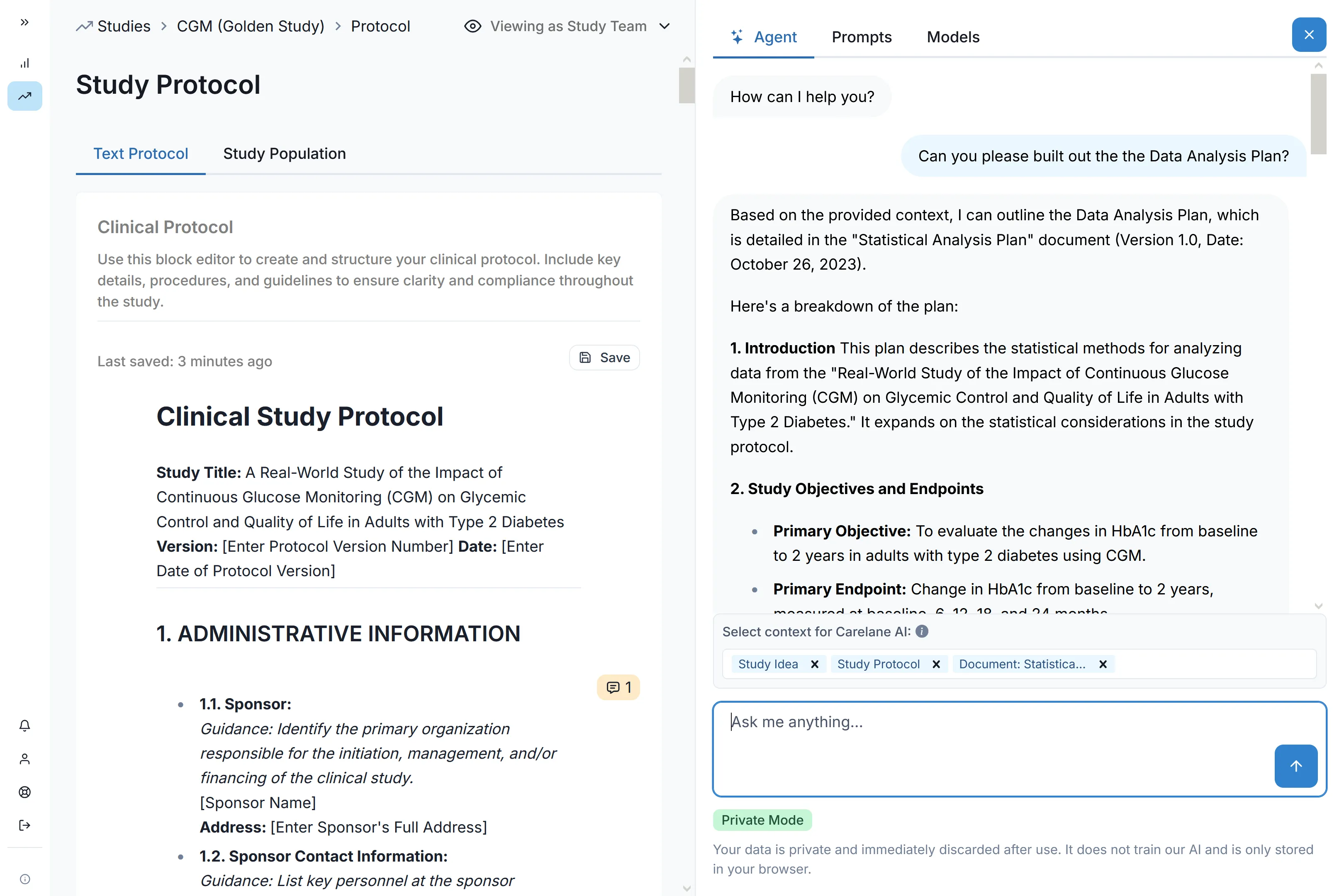
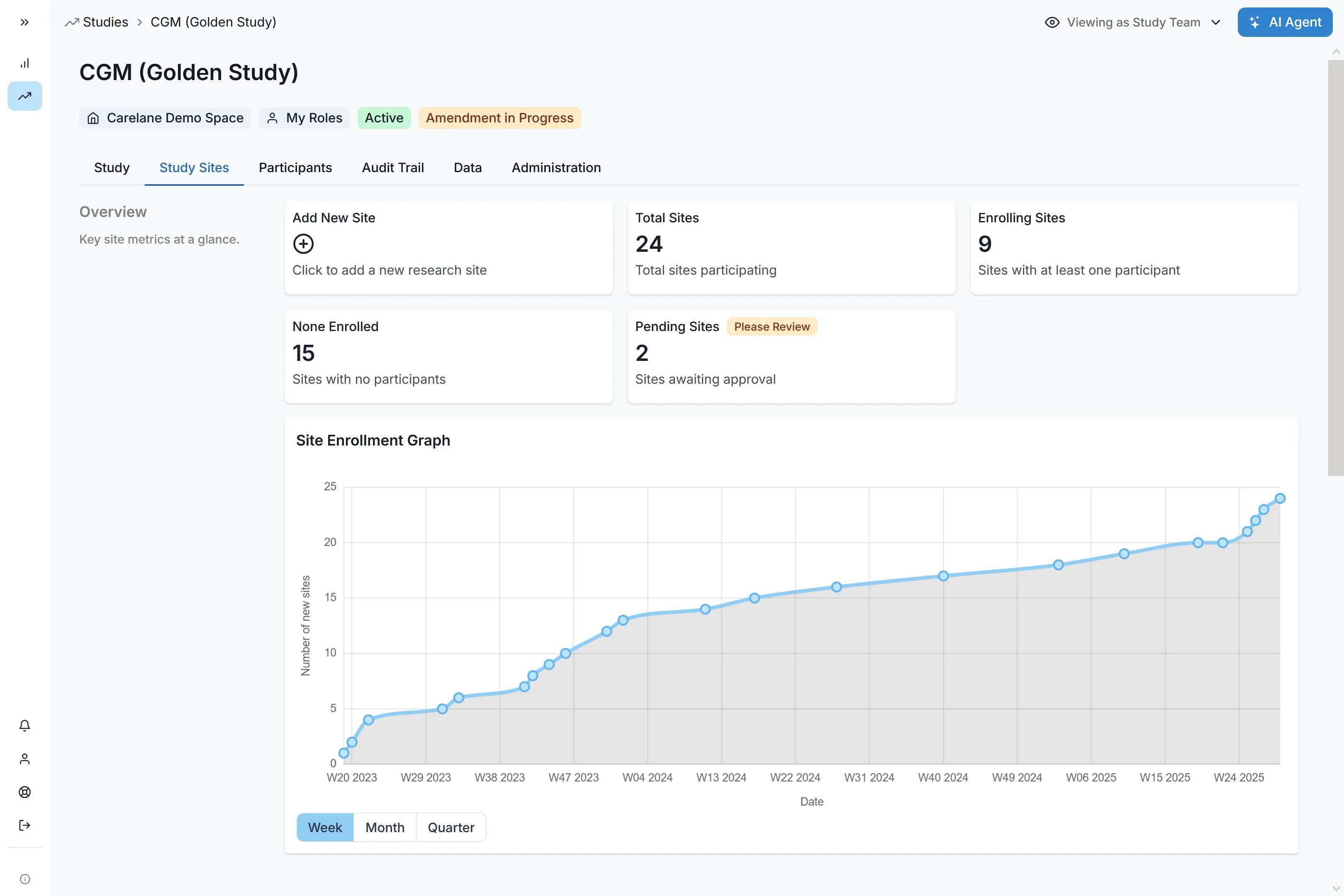
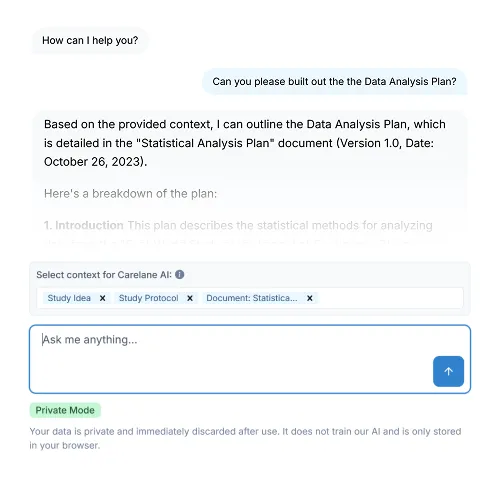
Carelane provides institutional-quality infrastructure for investigator-initiated trials, designed for maximum speed and efficiency. With AI-powered protocol digitisation, researchers can reduce study setup and site activation time by up to 90%. This automated ecosystem eliminates administrative bottlenecks, allowing academic teams to launch faster and scale multi-site research without traditional cost or technical barriers.
The Barriers for IIT Teams
High-quality research demands high-quality tools. Traditional clinical trial systems create unnecessary obstacles that prevent important research from moving forward.
Purpose-Built Infrastructure for Research Teams
Carelane delivers enterprise-grade capabilities specifically designed for the unique needs and constraints of
investigator-initiated research.

AI-Powered Protocol Digitisation
Transform your protocol document into a complete study system with 60-85% time savings on CRF design and EDC setup. Our AI CRF Builder automatically structures data elements and builds validated data collection forms—eliminating months of manual configuration and technical programming.

Streamlined Site Activation
Deploy multi-site studies at unprecedented speed. Carelane enables research teams to activate up to 60 sites in a single week, including capability assessment, feasibility evaluation, and complete onboarding.

Automated Operations That Scale
Reduce operational burden by up to 95% across critical functions. Automated monitoring, query management and site management shift your team from routine manual tasks to strategic oversight—freeing resources for research activities that matter.
Transparent, Scalable Pricing
Begin at no cost and scale according to your study needs. Our pricing model eliminates upfront licensing fees, hidden costs, and vendor lock-in. Research budgets should support research, not software overhead (subject to fair usage policy).
Proven Performance Across Research Programs
Research teams are leveraging Carelane to accelerate timelines, expand reach, and maintain focus on scientific objectives.
President of European Confederation of Medical Mycology (ECMM), Medical University of Graz
Real-World Evidence is Reshaping Clinical Research
Regulatory bodies increasingly recognise real-world evidence in decision-making. Investigator-initiated research is uniquely positioned to generate this evidence, when equipped with appropriate infrastructure.
Regulatory Evolution
The FDA and international regulators now accept real-world evidence for regulatory submissions. Investigator-initiated trials can contribute meaningfully to the evidence base with proper data quality and infrastructure.
Resource Optimisation
Research funding remains constrained across institutions and grant mechanisms. Infrastructure costs should enable research, not consume limited budgets. Efficient systems allow resources to flow toward scientific activities.
Time Advantage
In rapidly evolving research landscapes, speed to publication matters. Extended setup timelines delay enrolment, compress follow-up periods, and risk addressing questions that become outdated before completion.
Ready to try Carelane?
The new standard for Sponsors, CROs and Institutions to run faster trials. Instant adoption. Human-in-the-loop AI. Zero compliance risk.