Efficient, Secure Participant and Data Management
Streamline your study with secure participant management and powerful data tools. Our platform speeds up workflows, protects privacy, and delivers real-time insights.
Impenetrable PHI Encryption
We secure your PHI with zero-knowledge encryption. Each piece of data has a unique key, isolated by study and site. Only authorised site members hold the keys - not Carelane, not sponsors.
Precision Access,
Perfect Control (RBAC)
We use Role-Based Access Controls (RBAC) to meticuously sculpt user's access on a strictly need-to-know basis. With Carelane you gain surgical control over data visibility.
Architectural Data Isolation
We architect unique, segregated data collections for every client, study, and site. Encryption keys are stored in a separate key-fortress, ensuring your information is uniquely secure.
“The system was very easy to use. This was the first system we did not need training for.”
Hippokration General Hospital

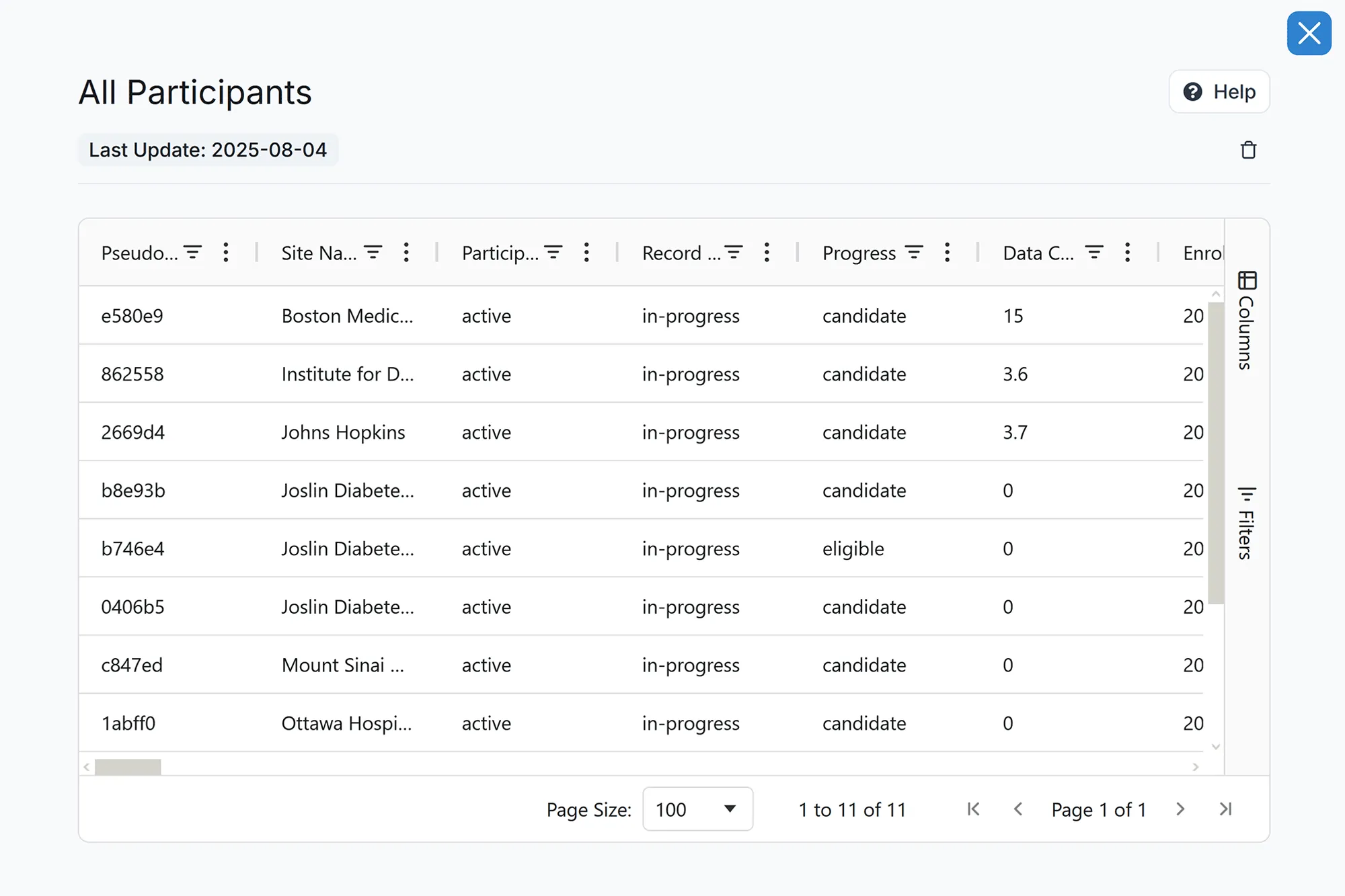
Participant Management
Manage prescreening, screening, and participant flow efficiently.
Tokenization/ Randomization
Preserve privacy by relying on our FIPS 140-2 encryption.
Data Capture & SDC
Facilitate flexible data exchange with FHIR and standard Clinical Terminology.
PHI Encryption
Protect sensitive PHI data, so they’re accessible only by authorized study site members.
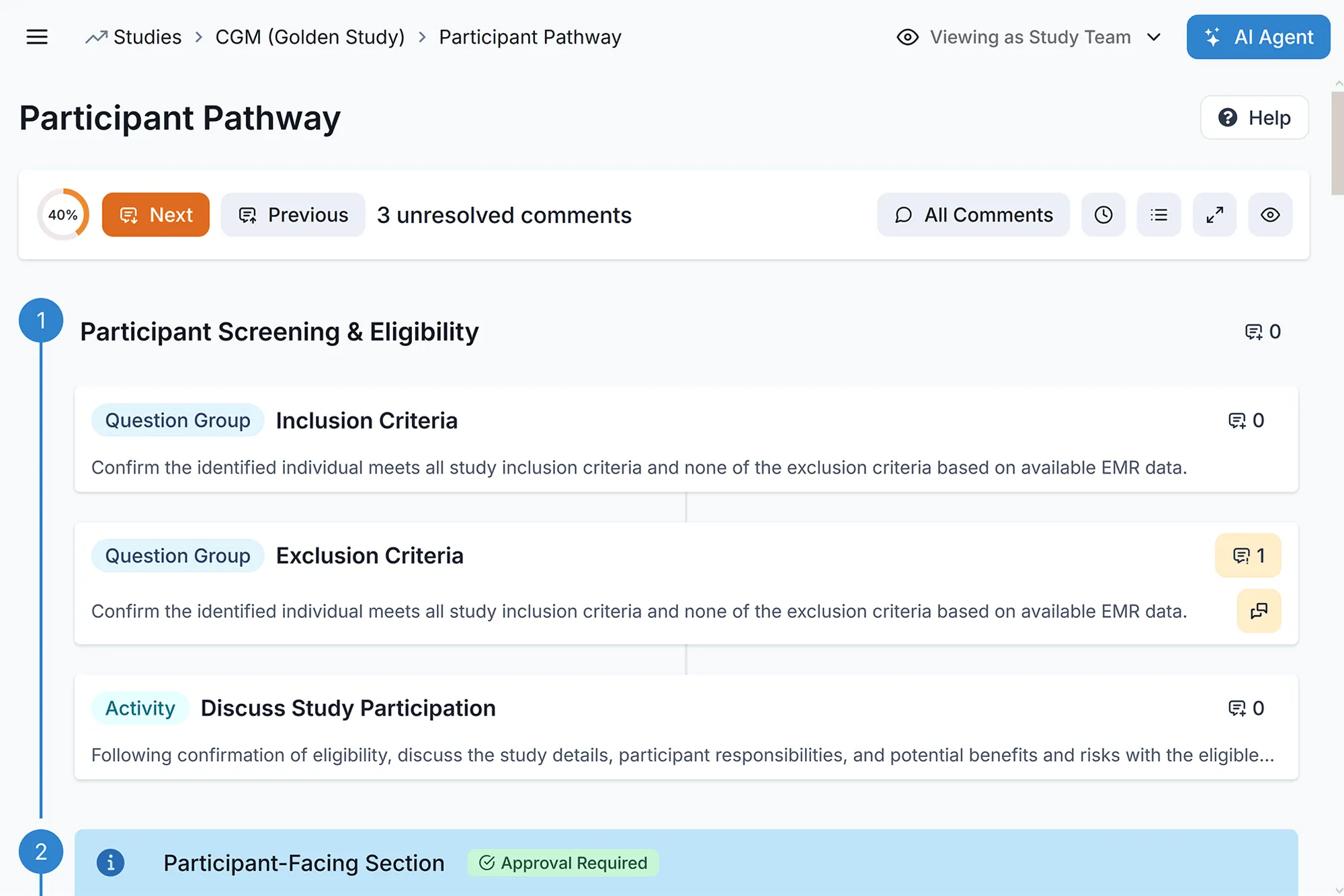
Embedded Protocols
Use FHIR workflow resources to align clinical care with study events.
Data Management
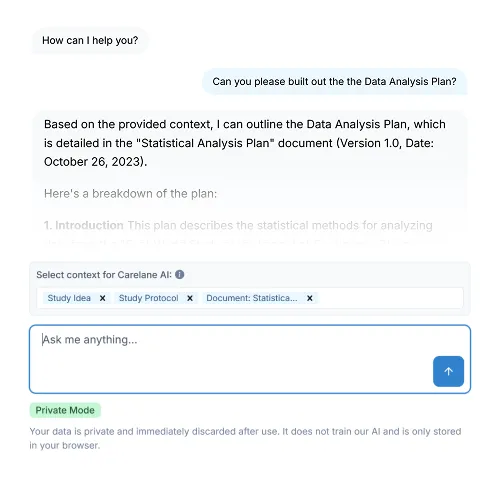
Use calculations, plausibility checks, built in data review processes, including AI supported query management, to track and review progress.
Remote Monitoring
Allow authorized monitors to assess data integrity with temporary access to PHI data.
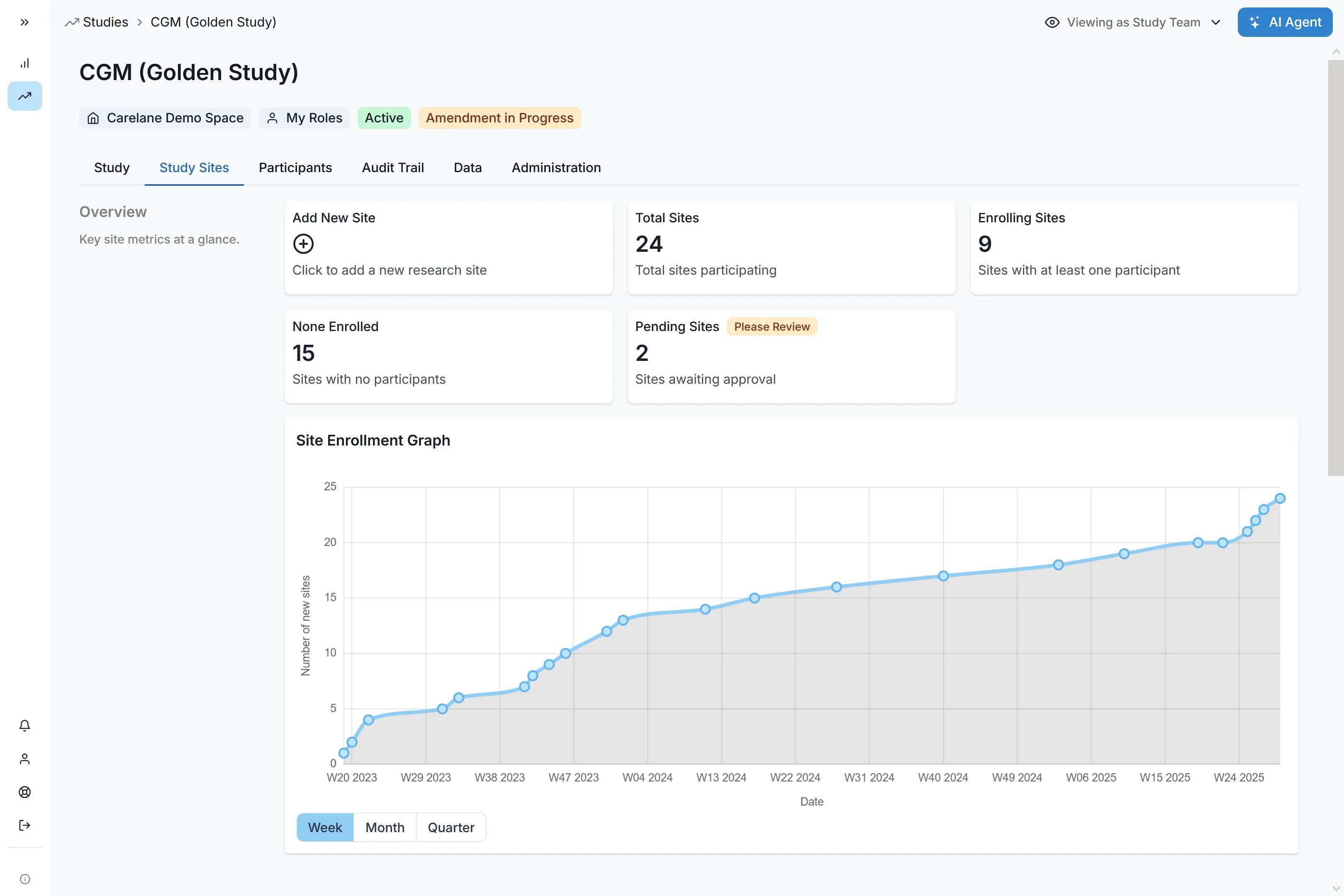
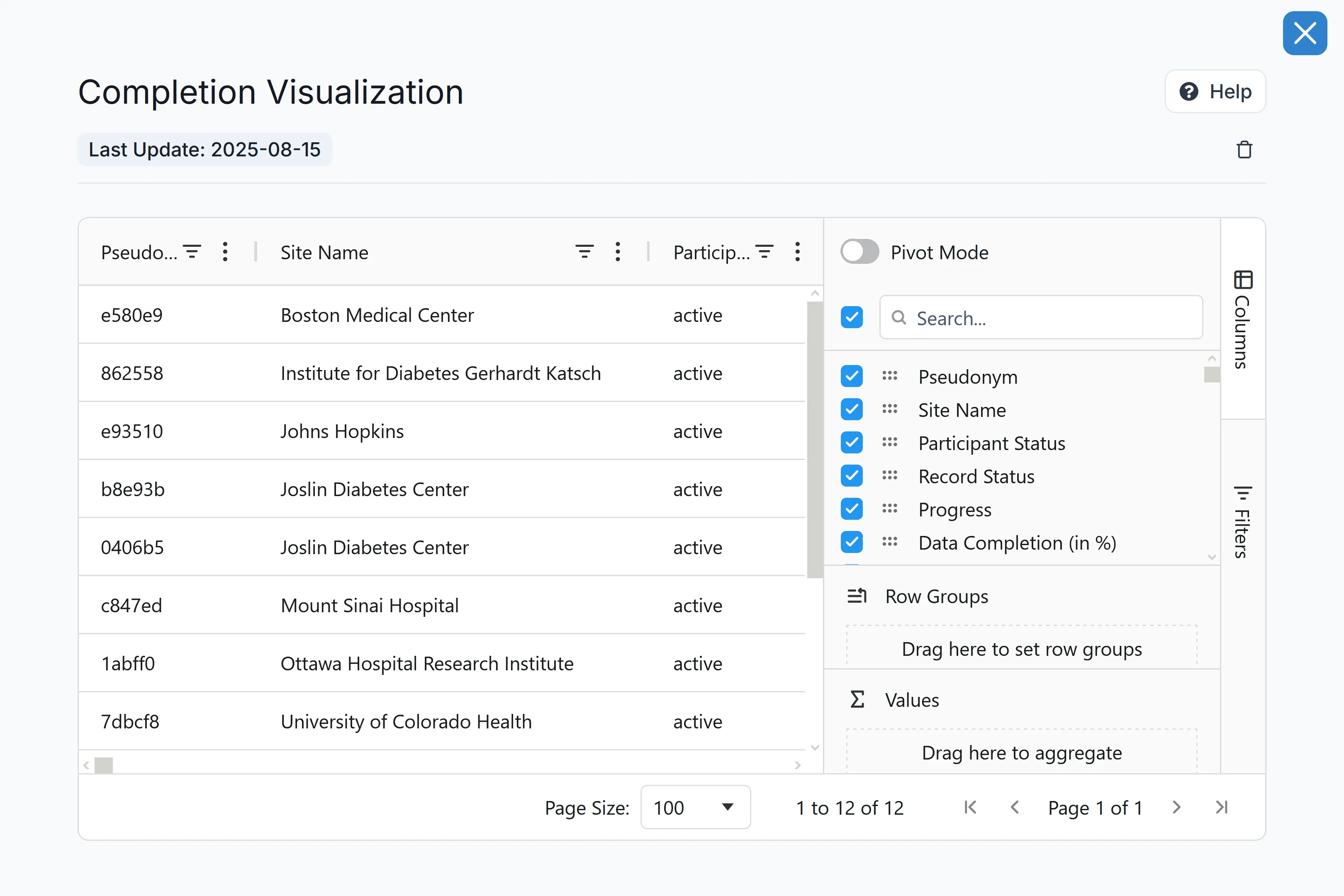
Reporting & Analytics
Use data dashboards to view KPIs, metrics, and data points for easy monitoring and decision-making. Integration with analytics platforms allows users to export data in native FHIR format for compatibility with existing analytics tools.
- eCOA
- ePRO
- eConsent
Ready to try Carelane?
The only end-to end solution for observational trials.
Unlock research at scale, slashing costs, complexity and friction!