Revolutionize Your Clinical Study Design with AI
Move beyond fragmented documents with our AI-powered platform. We digitize the entire clinical trial lifecycle, empowering you to design and execute studies with greater speed, clarity, and scientific rigor.
“The intuitive user interface simplifies and guides the protocol development process, removes many of the review and revision issues inherent in document based approaches, ensures all required information is obtained, and most importantly, enables medical and clinical staff to focus on the research goals and clinical aspects”
Protocol Development & Digitization
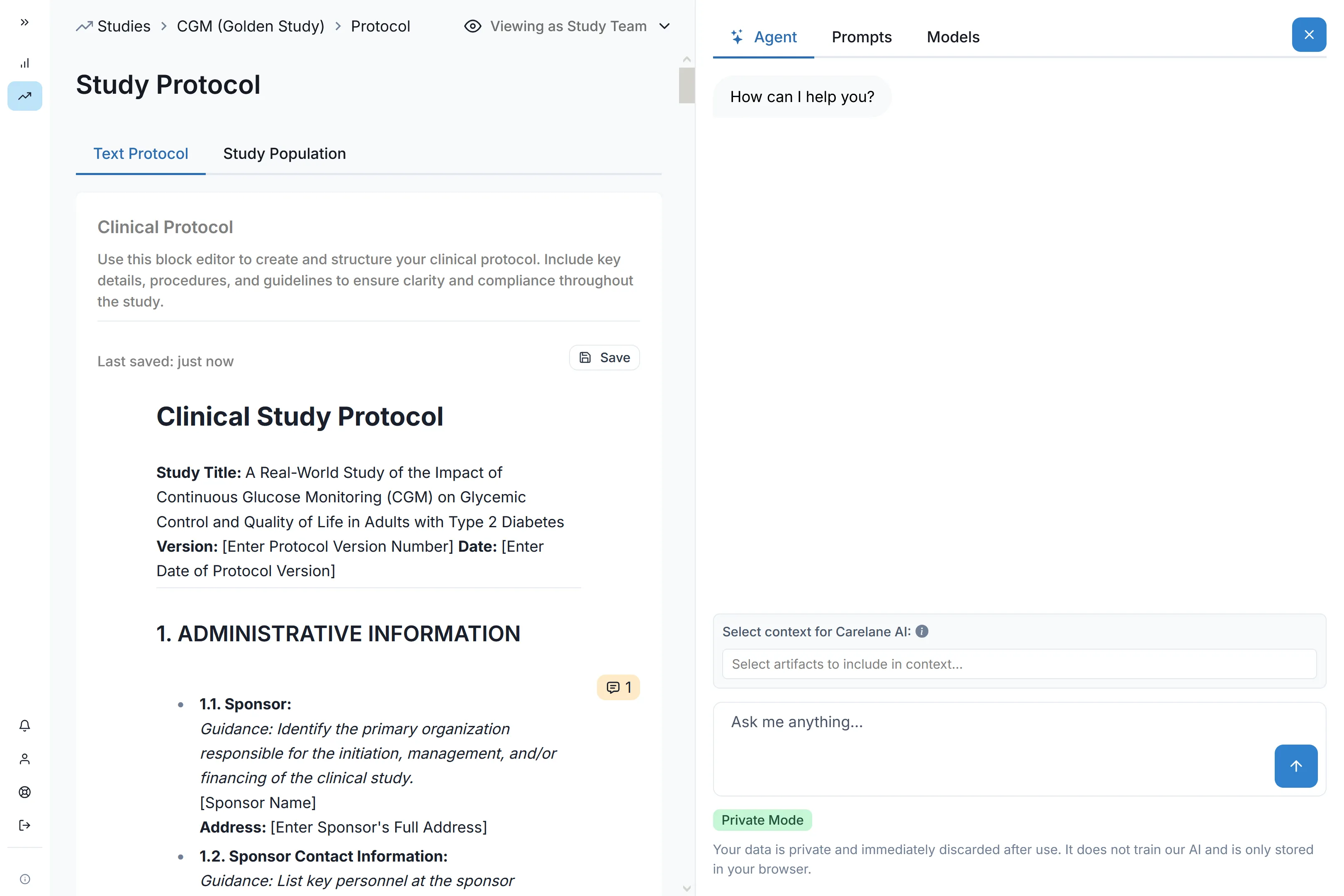
By structuring the digitized protocol; Our AI agent supports you in streamlining the entire clinical trial lifecycle—from study design to execution and analysis—by replacing fragmented, document-based processes with structured, digitized workflows. Simple prompt menus allow you to coordinate next steps and apply changes or queries.
- Evidence Classification
- Study Rationale
- Cohort Definition
- Endpoint Definition
Statistical Analysis Plan
Predefining and standardizing the statistical methods and procedures upfront, ensures transparency, reproducibility, and minimizes bias in the analysis. Carelane supports you all the way in creating this a critical document by drawing on key digitized artefacts such as the definition of the data collected data the definition of the study objectives. Make sure you don’t ever leave this hallmark of quality out of your study.
Schedule of Activities
Detail specific assessments and procedures and provide data collection timelines ensuring consistency and compliance.
AI CRF Builder
Standardize and optimize data collection for each participant with an intuitive interface for defining data elements and adding metadata.
Ethics/IRB Submission Ready
Carelane supports IRB workflow by centralizing essential study documents and streamlining communication between PIs and the IRB through a dedicated page and features designed to simplify the submission and review process, including guidance on study classification.
- Template Management
Ready to try Carelane?
The only end-to end solution for observational trials.
Unlock research at scale, slashing costs, complexity and friction!